-

Thermal radiation | By the end of the 19th century it was a common beliefs, that the physics is more or less complete, there were only a few open problems around. One of the open questions was the thermal radiation. In this experiment we verify that the thermal radiation obeys the Stefan-Boltzmann law.
-

Millikan experiment | J.J. Thomson is credited for the discovery and identification of the electrons. However Robert Millikan has measured the elementary electric charge as early as 1910. Millikan received the Nobel prize in 1923 for this experiment among others. In this lab work we apply the same method to measure the charge of the electron.
-

Activation potentials | In 1913 Niels Bohr presented his model in which the atom was a system consisting of a small, dense nucleus surrounded by revolving electrons —similar to the structure of the Solar System, but with attraction provided by electrostatic forces rather than gravity. In 1914, James Franck and Gustav Hertz performed an experiment which demonstrated the existence of excited states in mercury atoms, helping to confirm the quantum theory which predicted that electrons occupied only discrete, quantized energy states. In this lab work we reproduce their findings in the case of mercury and neon atoms.
-

Spectra of alcali metals | The word spectrum was introduced into optics by Isaac Newton, referring to the range of colors observed when white light was dispersed through a prism. Atomic spectroscopy is the study of the electromagnetic radiation absorbed and emitted by atoms. Atoms of different elements have distinct spectra and therefore atomic spectroscopy allows for the identification and quantitation of a sample's elemental composition. A comprehensive explanation of
the hydrogen spectrum was an early success of quantum mechanics.
-

Zeeman effect | The optical Zeeman effect is the effect of splitting a spectral line into several components in the presence of a static magnetic field. In 1896 Pieter Zeeman measured the splitting of spectral lines by a strong magnetic
field, a discovery now known as the normal Zeeman effect, for which he won the 1902 Nobel Prize in Physics shared with Hendrik Lorentz. In this lab work we observe the Zeeman splitting of the blue spectral line of mercury | The setup of
the experiment
-
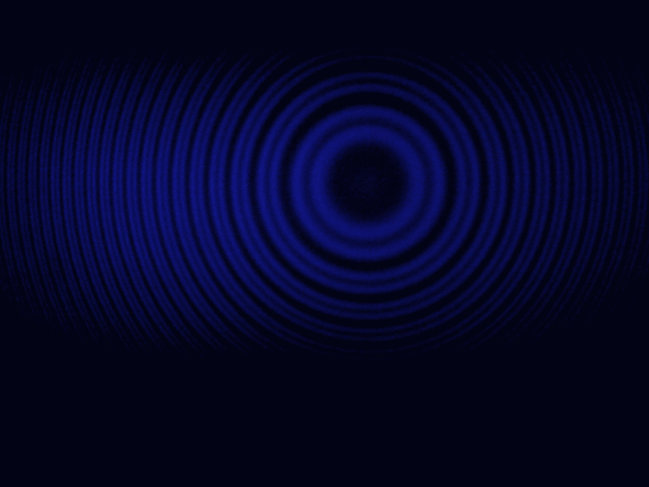
Zeeman effect | The optical Zeeman effect is the effect of splitting a spectral line into several components in the presence of a static magnetic field. In 1896 Pieter Zeeman measured the splitting of spectral lines by a strong magnetic
field, a discovery now known as the normal Zeeman effect, for which he won the 1902 Nobel Prize in Physics shared with Hendrik Lorentz. In this lab work we observe the Zeeman splitting of the blue spectral line of mercury | The splitting
of the blue spectral line of mercury observed with Fabry-Perot interferometer
-

X-ray flourescence (XRF) | XRF is a widely used non-destructive, nuclear analytical spectroscopy tool, which allows to determine the elemental composition of materials. XRF analyzers determine the chemistry of a sample by measuring the
fluorescent (or secondary) X-ray emitted from a sample when it is excited by a primary X-ray source. In this laboratory, we identify several samples and also confirm the validity of the Moseley law. | Our XRF equipment
-

X-ray flourescence (XRF) | XRF is a widely used non-destructive, nuclear analytical spectroscopy tool, which allows to determine the elemental composition of materials. XRF analyzers determine the chemistry of a sample by measuring the fluorescent (or secondary) X-ray emitted from a sample when it is excited by a primary X-ray source. In this laboratory, we identify several samples and also confirm the validity of the Moseley law. | Various samples in the storage drawers
-

PET (Positron Annihilation) | In 1928, Paul Dirac published a paper proposing that electrons can have both a positive and negative charge. In 1932 Carl David Anderson discovered the positron, by identifying an ion trail appeared on the
photographic plate with a curvature matching the mass-to-charge ratio of an electron, but in a direction that showed its charge was positive. One year later positron annihilation has been experimentally confirmed. Both Dirac and Anderson
received the Nobel prize for their achievements. In this laboratory we investigate the position and size of an ideal tumor in the subject.
-

Spectrophotometry | Visible and ultraviolet spectroscopy is routinely used in analytical chemistry for the quantitative determination of different analytes, such as transition metal ions, highly conjugated organic compounds, and biological macromolecules. Spectroscopic analysis is commonly carried out in solutions but solids and gases may also be studied. In this laboratory we measure the equilibrium constant of reaction by measuring the absorption spectra of a series of solutions with different concentration of solvents. | Shimadzu UV-2101PC spectrophotometer
-

Spectrophotometry | Visible and ultraviolet spectroscopy is routinely used in analytical chemistry for the quantitative determination of different analytes, such as transition metal ions, highly conjugated organic compounds, and biological macromolecules. Spectroscopic analysis is commonly carried out in solutions but solids and gases may also be studied. In this laboratory we measure the equilibrium constant of reaction by measuring the absorption spectra of a series of solutions with different concentration of solvents. | The prepared solutions to measure: the darker violet the solution is, the more complex it contains.
-
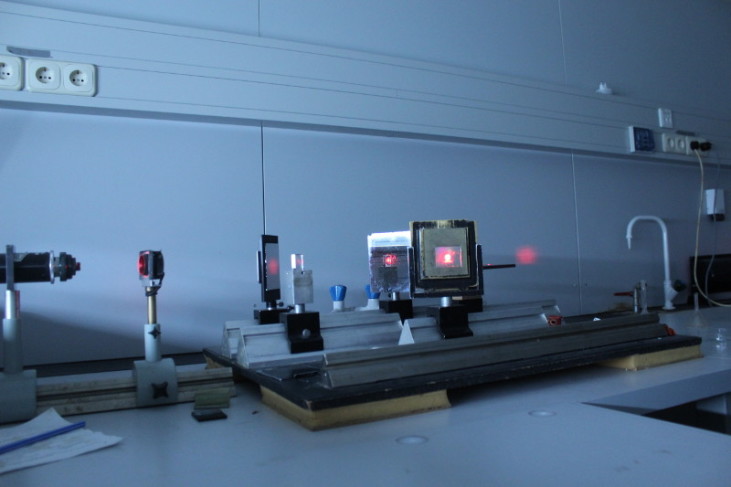
Holography | In this laboratory we learn the basics of the traditional (analog) holography by making three small size holograms. Despite that holograms are developed in similar manner than analog photos, the holographic method enables us
to reproduce the full light field thus making the image of the object much more realistic. Dennis Gabor received the Nobel prize for his discovery in 1971. | The holograms made in this laboratory are visible only under laser light in similar circumstances.
-

Holography | In this laboratory we learn the basics of the traditional (analog) holography by making three small size holograms. Despite that holograms are developed in similar manner than analog photos, the holographic method enables us
to reproduce the full light field thus making the image of the object much more realistic. Dennis Gabor received the Nobel prize for his discovery in 1971. | Photo of a hologram of a tiny locomotive model
-
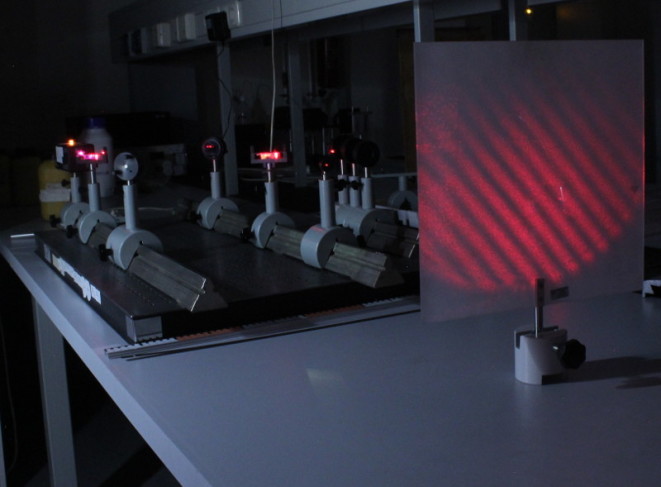
Quantum Eraser | In variants of double-slit experiment it is well-known that when action is taken to determine which slit a photon has passed through, the "marked" photon cannot interfere with itself. In the quantun eraser experiment we
"unmark" the previously "marked" photons to regain the interference fringes. In this laboratory we perform the analogous classical experiments which can be explained in the framework of classic electrodynamics. | The Mach-Zehnder interferometer with the typical interference fringes in the front
-
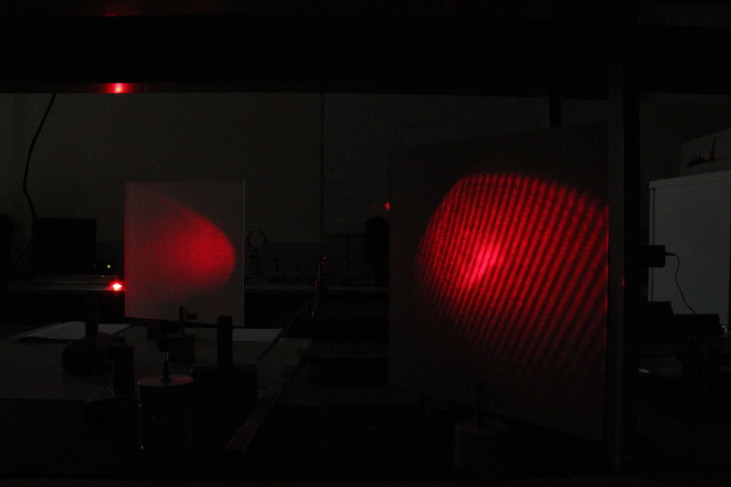
Quantum Eraser | In variants of double-slit experiment it is well-known that when action is taken to determine which slit a photon has passed through, the "marked" photon cannot interfere with itself. In the quantun eraser experiment we
"unmark" the previously "marked" photons to regain the interference fringes. In this laboratory we perform the analogous classical experiments which can be explained in the framework of classic electrodynamics. | There is no interference
on the left screen due to the "marked" photons, however on the right screen the interference fringes are restored by inserting the additional 3rd polarization filter which acts like a quantum eraser.
-
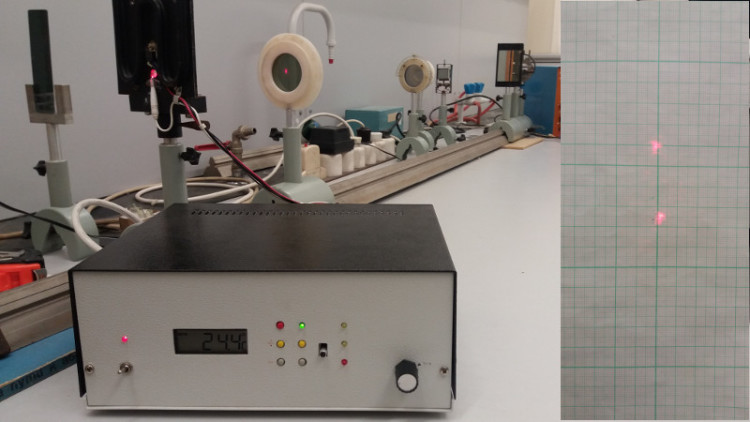
Liquid crystals and LCDs | We use devices equipped with LCDs on a daily basis. Their advantages in comparison with other techniques (e.g. cathode ray or plasma screens) are obvious as they are slimmer, lighter, consuming less energy and
they are getting also cheaper lately. In this laboratory we learn the basics of the liquid crystals and liquid crystal displays. | Birefringence in liquid crystal prism: by increasing temperature a liquid crystal-liquid phase transition
can be observed.
-
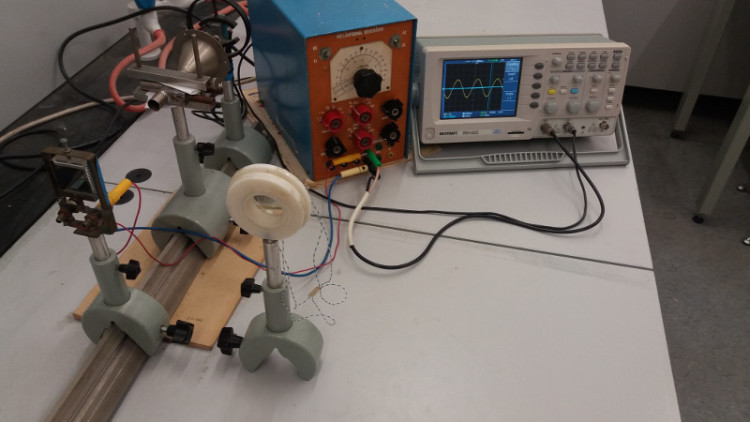
Liquid crystals and LCDs | We use devices equipped with LCDs on a daily basis. Their advantages in comparison with other techniques (e.g. cathode ray or plasma screens) are obvious as they are slimmer, lighter, consuming less energy and
they are getting also cheaper lately. In this laboratory we learn the basics of the liquid crystals and liquid crystal displays. | Characteristics of two different basic types of LCDs are studied by the means of laser diode, photo diode,
wave generator and oscilloscope.
-

Granular materials | Granular materials are commercially important in applications as diverse as pharmaceutical industry, agriculture, and energy production. The constituents of granular material are large enough such that they are not subject to thermal motion fluctuations, therefore the standard methods of statistical mechanics can not be applied to their description. For example, the granular materials do not follow the simple formula of hydrostatic pressure but follow a saturation curve as predicted by Janssen. In this laboratory we test the Janssen modell.
-

Charge to mass ratio of electron | J.J. Thomson in 1897 was the first to suggest that one of the fundamental units was more than 1,000 times smaller than an atom, suggesting the subatomic particle now known as the electron. By comparing
the deflection of a beam of cathode rays by electric and magnetic fields he obtained robust measurements of the mass-to-charge ratio. Assuming that the charge of the electron is the elementary charge he could calculate the mass of electron. In this lab work we measure the mass-to-charge ration of electron with the experimental setup of J.J. Thomson.








































